Dose Dense Trial
 Dose Dense Protocol
Dose Dense Protocol
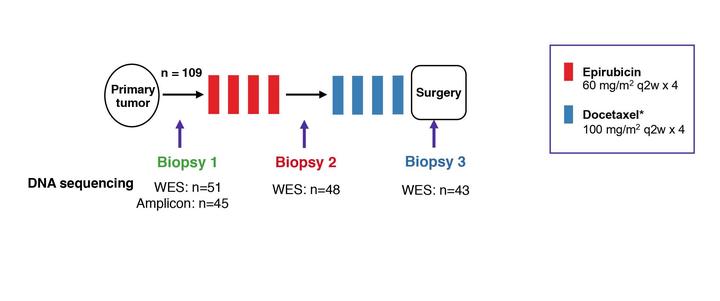
The scientific aim of this study is to explore mechanisms of resistance to chemotherapy in breast cancer. To do so, we explore molecular parameters predicting response to chemotherapy administered prior to local therapy in large, primary breast cancers. Neoadjuvant (primary medical) therapy has got wide acceptance as primary therapy in breast cancer. In addition, this treatment provides an optimal setting studying the mechanisms of drug resistance in human cancers. Considering chemotherapy for primary breast cancer treatment, contemporary trend has been to treat these tumors more aggressively. High-dose therapy involving stem cell support is not advocated, as this has not been shown to improve long-term survival in early breast cancer. However, the attitude in general has been toward a more aggressive approach within the frame of “conventional” therapy. Based on theoretical modeling, an alternative approach, “dose-dense” therapy, has been advocated. Recently, that concept was brought to the test in two adjuvant trials. Thus, Citron et al applying doxorubicin, paclitaxel and cyclofosfamide revealed an improved outcome for dose-dense (2-weekly) administration compared to regular 3-weekly scheduling. In contrast, the German GEPARDUO study reported doxorubicin plus cyclophosphamide and docetaxel, given in sequence on a 3-weekly basis (8 cycles), to be superior to doxorubicin and docetaxel given in concert on a 2-weekly basis for 4 cycles. However, the doses administered (doxorubicin 50 versus 60 mg/m2; docetaxel 75 versus 100 mg/m2) was unequal, meaning total drug dose exposure differed between the two treatment arms. While more data are warranted, a reasonable interpretation of available data suggest sequential administration of different compounds in a dose-density approach to be a suitable regimen provided adequate total doses are given. Treatment regimen Each patient will receive 4 cycles of epirubicin 60 mg/m2 given at 2 weekly intervals together with G-CSF. Thereafter, docetaxel 100 mg/ m2 will be given at 2 weekly intervals for 4 cycles. Patients revealing positivity for HER2 status will have trastuzumab (Herceptin) implemented together with docetaxel but not during anthracycline treatment. Response evaluation Response will be evaluated based on clinical examination and MRI, each assessment to be done separately.
Clinical examination will be performed prior to commencing therapy (before surgical biopsy) and subsequently at 4, 6 and 8 weeks on therapy. Response will be classified according to the common “RECIST” criteria. An important exception is to be made. As argued in a previous protocol, we consider the RECIST definition of “progressive disease” as a 20% increase in the sum of the largest tumor diameters to be too liberal with respect to large primary tumors. Based on experience in our clinic, we believe the definition of progressive disease as an increase of > 25% in the product of the largest tumor diameter and its perpendicular (the previous common UICC criteria) to be a more suitable definition, protecting patients from undergoing deterioration of their clinical condition. This is in accordance to our previous experience. In case of “progressive disease” at any stage during epirubicin treatment, the patient will terminate epirubicin immediately and go ahead with docetaxel treatment. In case of progressive disease on docetaxel treatment, further therapy is left to the physician’s discretion. Considering MRI assessment, this should be performed prior to commencing therapy, in the interval following the 4th cycle of epirubicin (prior to commencing docetaxel) and after the 4th cycle of docetaxel, prior to surgery. Surgery While many centers practice breast conservative surgery for tumors successfully downstaged by primary medical treatment, in general we have applied a conservative approach, advocating mastectomy. However, downstaging for limited surgery is not a primary or secondary endpoint of this study. In general, we will advocate mastectomy also for patients with a clinical complete response. However, this practice may change based on contemporary results from other centers, and the protocol allow limited surgery at the physicians discretion in individual patients. The area of molecular biology is rapidly developing with respect to biological knowledge as well as technical analytical methods. Thus, it is not possible to predict upfront which genes may be of particular interest in 5 years from know; neither is it possible to foresee completely which laboratory methods will be available. Our aim is to explore potential genetic alterations explaining the mechanisms of drug resistance. While it is not possible to predict in detail, the aim of the study and all analysis to be conducted should aim at this major goal.